Back to School: Part 2 - The Basics of IVF
The backstory: a brief history lesson
In vitro fertilization (IVF) as a fertility treatment in humans is possible only because scientists have studied natural processes in great depth. Using what they knew about animals systems, the pioneers in human reproduction were initially animal reproductive biologists. Robert Edwards, Ph.D., began his work at Cambridge with experiments in mice.
He learned on animal models to use drugs to induce superovulation. Others used rabbits to explore the intricate processes of fertilization while dreaming of using their knowledge to someday treat infertility in humans. Scientists knew that each animal model had different conditions for in vitro embryo development, and the specific fluid conditions of the human tube were studied for years and chemically replicated.
IVF was performed in human subjects by Dr. Edwards and gynecologist Patrick Steptoe, MD, in the 1970s more than 300 times before baby Louise Brown was conceived. At this time, every egg retrieval was done by laparoscopy. Dr. Edwards would go on to win a Nobel Prize for his pioneering of IVF. Not receiving enough credit was the nurse who managed all of the initial patients, Jean Purdy, RN.
After initial success, physicians and researchers studied and further improved IVF processes. Thanks to successful efforts in Australia, where the team was already in human trials, IVF quickly became applied successfully in women who had been trying to conceive for a variety of reasons. In the first decades of IVF therapy, major advancements happened relatively quickly. Thanks to Alan Trounson, Ph.D., in Melbourne and Georgianna Seegar Jones in Norfolk, drug stimulation methods improved and bececame more aggressive. The first frozen embryo and frozen egg pregancies made headlines. None of these advances would have been possible without the leaders’ knowledge of the relevant biology.
Thanks to ongoing research, current patients trying to conceive through IVF or IUI have enormous advantages over patients of previous decades. Research goes on every year into the intricacies of natural processes, as well as on improved methods of diagnosis as well as treatment for infertility patients. Understanding natural human processes will help you understand the rationale for each step of the various stages of IVF treatment.
Ovulation in nature
In part one, we reviewed how ovulation occurs in nature. In part two, we’ll be discussing IVF background and processes.
Superovulation in IVF: the origins of gonadotropins
The concept of superovulation was established in 1957 in mice by English scientist Ruth Fowler and colleagues. Animal gonadotropins (“gonad” means “grow,” from the Greek) were injected to produce multiple eggs, which were retrieved from the tubes of the mice.
The company seeking to make follicle stimulation hormone (FSH) for ovulation needed a human source for the hormone. After menopause, FSH levels are very high and it is excreted in the urine. A drug tailored for humans was produced by chemically extracting gonadotropins from the urine of menopausal women. The first to collect urine for commercial production of human menopausal gonadotropin (HMG) were menopausal nuns in Italy. Urine was collected into tanks and transported to a chemical extraction plant by a company later to be known as Serono. It took almost a month for one nun to produce enough for extraction of the drug to supply one patient for a cycle of treatment.*
Initally the compounds were developed for use by women who needed drug stimulation to ovulate. Now they are used for this as well as for insemination treatment and are the dominant drug used in IVF. As the need for FSH as a drug grew, gathering the required amount of urine became a logistical nightmare.
FSH is a complex molecule that can’t be made by synthesis in a factory. Biotechnology allows pharmaceutical production of pure FSH by recombinant DNA methods, involving hamster ovaries. The DNA of the animal cells is altered by recombinant technology so that they produce the FSH hormone.
Gonadotropins in modern – day conventional IVF
In conventional IVF, HMG or FSH or a combination of both are typically used daily starting days 2 to 3 of the menstrual cycle to override the natural inhibition of the dominant follicle. The doses of the FSH hormone compared to the natural levels produced by the pituitary represents a massive overdose. Accordingly, many smaller follicles emerge and grow as a group or cohort of follicles of varying sizes. This is seen easily on transvaginal ultrasound during IVF monitoring visits. The largest follicles reach 18mm or more, signaling they are approaching ovulation.
At each IVF clinic monitoring visit, the amount of estrogen is measured by blood testing, as it rises continually. Commonly, it will reach 1000 pg/ml. Multiples of this number can occur when 15 to 20 or more follicles are growing. You may recall from part one that in nature, estrogen is measurable in the blood with a max of around 300pg/ml. If you have low estrogen levels and low numbers of follicles, the number and quality of eggs becomes a concern, while extremely high levels can reflect a risk of ovarian hyperstimulation syndrome. Your IVF team will discuss their concern if this risk increases.
The inner workings of egg development
In stimulated cycles, the eggs themselves develop the same way you read about in part one. As we mentioned there, scar tissue from surgery, endometriosis, or infection involving the tube or ovary can cause impairment of these delicate processes. The most difficult are those which completely block the tubes.
IVF – the trigger shot
In an IVF cycle, selecting a “trigger shot day” is a critical decision. Around the ninth to twelfth day of daily medication, the cohort of follicles reaches the ideal time. One or more follicles are over 18 mm, and there is usually a good crop of medium-size follicles. A decision is made to trigger ovulation and set a time for the retrieval – an exciting pivotal event in the intense process of monitoring IVF.
The trigger shot given is HCG, a hormone which has been traditionally derived from the placenta. The HCG has the same action as the LH surge, completing the final development of the egg and preparing the follicles for ovulation. Commonly this HCG is administered as Ovidrel, the trade name for a recombinant form of HCG.
Egg retrieval
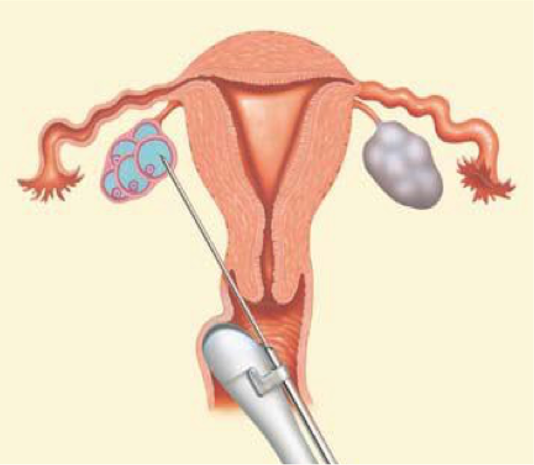
In the IVF process, we must retrieve the eggs before they are ovulated, around 34 to 36 hours after the HCG trigger. Under ultrasound guidance, the needle passes through the vagina and is inserted into the follicle.
Typically, the egg is retrieved from the larger follices. In the smaller follicles particularly, less mature eggs may persistently adhere to the side of the follicle and not come out. Rarely do all the follicles yield an egg – for example, if you have 15 follicles aspirated, nine eggs might be retrieved.

As the follicles are aspirated under ultrasound guidance, the embryologist is in the next room working with a microscope under sterile, rapidly circulating air looking at the follicular fluid as it comes in from the operating room. Fertilization out of the body was performed in animals years ago literally in vitro, in glass test tubes. Now the glass test tubes have been replaced by specially designed plastic Petrie dishes.
On a warm surface, the embryologist quickly searches the retrieved follicular fluid. The egg is a tiny dot within the cumulus mass.
On high magnification a few hours later, the embryologist can identify the mature eggs with a large, visible second polar body. These eggs have 23 chromosomes and are prepared for fertilization – acceptance of the 23-chromosome packet from the sperm. The embryologist notes that these are metaphase II (MII) eggs, meaning the polar body is present.Your IVF data sheet likely designates these eggs as ideal.
Some eggs will be immature, MI, and some will be too immature, dark, and nonviable. Every batch of eggs will have several immature eggs. Most of them will not contribute to producing embryos, but a few do, so they are observed and fertilized later when appropriate.
When we see MII eggs, we still understand that they are not always perfect. Aside from maturity of the nucleus, the cytoplasm of the egg must be competent. That is not always the case, and there is no way to see this before ICSI is done. Only the next day, when we see how many fertilize, will we know which ones are potentially able to produce a good embryo.
Sperm prep for IVF

Semen is obtained by masturbation, surgery, or thawed from the partner or donor on the day of the egg retrieval.
It is diluted, and its prep involves separating the seminal fluid. For conventional IVF, the sperm are counted so as not to introduce too many into the dish at once.
Fertilization in nature and IVF
The sperm fertilizes the egg in vitro (“in glass”) the same way it fertilizes in vivo (“in body”). Part one covers the process of the sperm penetrating the egg in detail.
In vitro fertilization

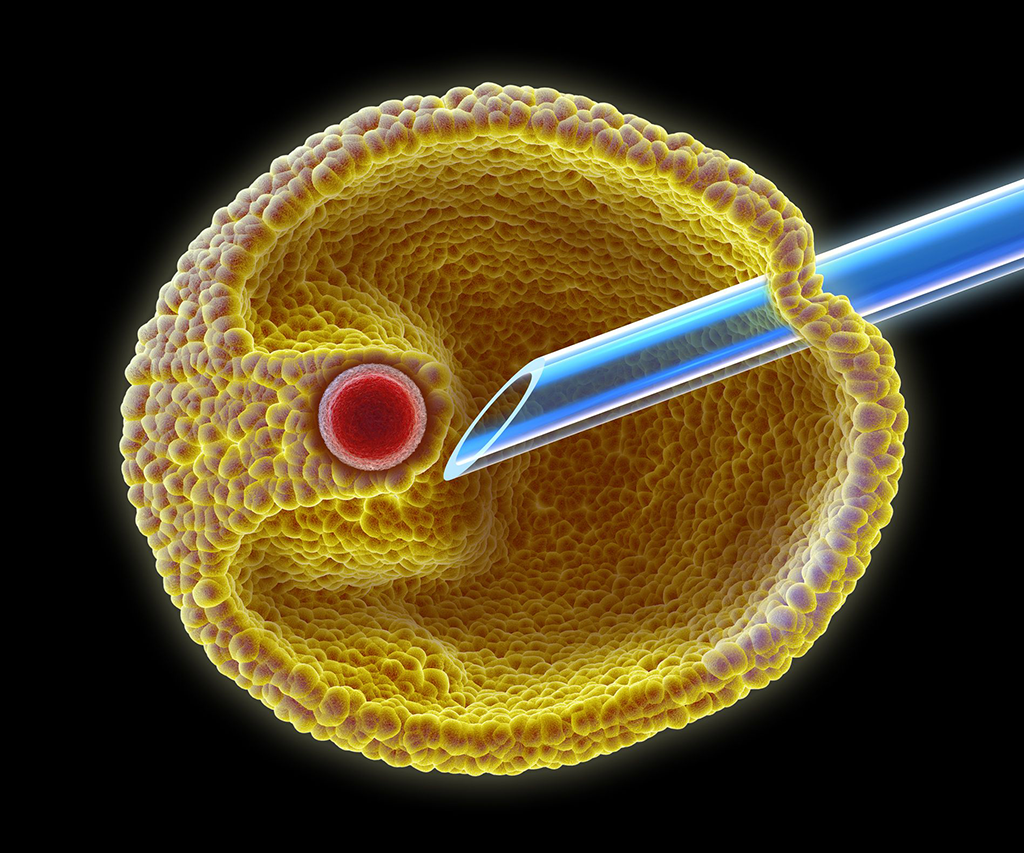
Now it is time for the sperm and egg to begin a process of fusion. First, the sperm are diluted so that the number is relatively small. In conventional IVF, this process is carried out by a measured number of sperm being placed into a dish about five hours after egg retrieval, allowing for a bit more maturation of the egg.
In cases of low sperm numbers, intracytoplasmic sperm injection (ICSI) is used. This guarantees that a sperm will be inserted into the egg.
Specialized media are used to nourish the eggs and developing embryos.
Embryo development
Once the sperm enters the egg naturally or with conventional IVF or by ICSI, the process is the same. You can refer to part one to understand the intricate details of what takes place in this early part of embryo development. The main difference when doing IVF is that it can all be seen under a microscope.
The morning after the IVF retrieval, the eggs are inspected. Ideally two pronuclei are seen, the nucleus of the sperm and the now visible nucleus of the egg. This allows the embryologist to announce that fertilization has occurred.
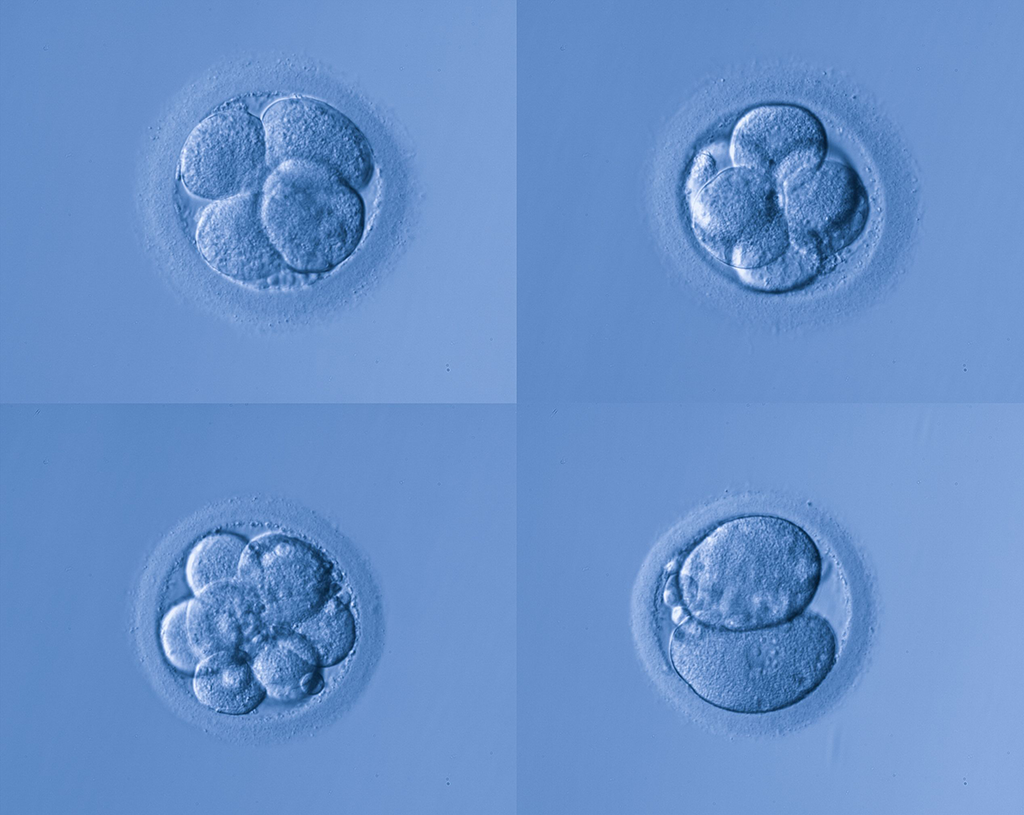
Later that day, the one-cell embryo known as a zygote then begins division into two cells. Real-time observation of the exquisite cell division process is sometimes carried out under observation in the incubator by sophisticated imaging systems and computer analysis. Flaws in the process can be observed and have been linked to ability to predict the outcome of a particular embryo.
After fertilization, there is a five- to six-day period of incubation until the embryo is 80 to 100 cells and is considered a blastocyst. The incubator is set at body temperature. Embryos are disturbed as infrequently as possible. The amount of oxygen in the air in the incubator is controlled and is lower than the atmosphere. This mimics the environment in the fallopian tube as well, and reduces oxidation and cell damage.
IVF embryo development: the history of IVF media
IVF in humans was successful thanks to the animal IVF which preceeded it. Animal reproductive specialists knew that each animal species had different chemisty in the tubes where fertilization occurs. Samples were taken of human tubal fluid and special media was developed to mimic the findings.
In IVF, this media surrounds the cells during the fertilization process and during embryo development. The first IVF efforts in the early 1980s relied on customized batches of media in every lab. A few years later, commercial companies standardized the media. This development has been key to allowing IVF to be successful all over the world.
Assisted hatching in IVF
After approximately five days, the embryo, now called a blastocyst, leaves the fallopian tube and travels down to the uterus where it “hatches” out of the outer shell of the egg called the zona pellucida just before implantation.
In certain situations, the zona is overly hardened artificially, which can inhibit hatching of the egg and implantion, as those embryos would be impaired. In IVF, the last manipulation of the embryo before transfer is often the making of a small hole in the zona. Assisted hatching is done minutes before the embryo transfer. The most precise method to do this is with a microscopic laser.
Embryo transfer
It’s transfer day! This is always an exciting, albeit nerve-wracking day in the IVF process. An embryo transfer catheter is loaded with the embryo. Using ultrasound for accuracy, your doctor passes a catheter through the cervix into the uterus. Once the tip of the catheter is in the desired location, your fertility doctor transfers the embryo into the uterus. After the catheter is gently removed, it is always checked under a microscope to confirm the embryo was transferred. This process requires no anesthesia and you can resume normal activity that day.
The two-week wait
After transfer, there is a two-week wait until your fertility specialist does a pregnancy test to discover if the embryo implanted and is growing into a healthy pregnancy.
Egg factors and IVF failure
As we know, in nature as well as in IVF, pregnancy is not the result every time fertilization occurs. There are several reasons why. Below are two reasons that occur because of issues with the egg.
Chromosomal errors
Fertilization may occur, but the embryo may arrest in development if there is a major genetic error in the nucleus of the egg. Around half of the time, abnormal genetic complement in the egg before the sperm even arrives is really the problem. The only way to know if this is occurring is to wait for the blastocyst, perform embryo biopsy, and freeze the embryos. Chromosome analysis can be performed on a few cells from the embryo. This giant leap in IVF technology then allows selection of only chromosomally normal embryos for transfer.
Cytoplasm
If the cytoplasm of the egg, called ooplasm, has not matured, cell division will fail somewhere in the fertilization or early development process. Subtle defects in the ooplasm may lead to arrest at any stage of development, so in the days after the initial IVF, there is attrition of the embryos. This is the same regardless of whether ICSI was used. For example, of seven fertilized eggs, you may have two to three embryos by the fifth or sixth day when they are ready for implantation. Of course there is a range of possible outcomes.
Key to successful development is the complement of mitochondria. Older patients have less of these energy-producing organelles than ideal. At this time, fertility specialists cannot diagnose this problem, but we know it is one of our barriers to success.
In conclusion
Hopefully, this two-part “Back to School” article clears up questions you may have about fertilization. Your fertility center lab is designed to mimic the natural process of fertilization as closely as possible. The advances in IVF since it began in humans in the 1980s have been significant in making the IVF process as natural as possible.
References
* Gosden, Roger. Let There Be Life: An Intimate Portrait of Robert Edwards and His IVF Revolution. Jamestowne Bookworks, 2019. Trebichalská Z, Holubcová Z. Perfect date-the review of current research into molecular bases of mammalian fertilization. J Assist Reprod Genet. 2020 Feb;37(2):243-256. doi: 10.1007/s10815-019-01679-4. Epub 2020 Jan 6. PMID: 31909446; PMCID: PMC7056734.
Palermo GD, O'Neill CL, Chow S, Cheung S, Parrella A, Pereira N, Rosenwaks Z. Intracytoplasmic sperm injection: state of the art in humans. Reproduction. 2017 Dec;154(6):F93-F110. doi: 10.1530/REP-17-0374. Epub 2017 Nov 20. PMID: 29158352; PMCID: PMC5719728.
About BUNDL Blog
The BUNDL Blog features need-to-know information for all aspects of the fertility journey, including info about IVF, IUI, and affording care.


